List and Describe Three Examples of Useful Oxidation Reactions
So the food that we consume is converted into energy by redox reactions only. H is reduced Oxidation number.
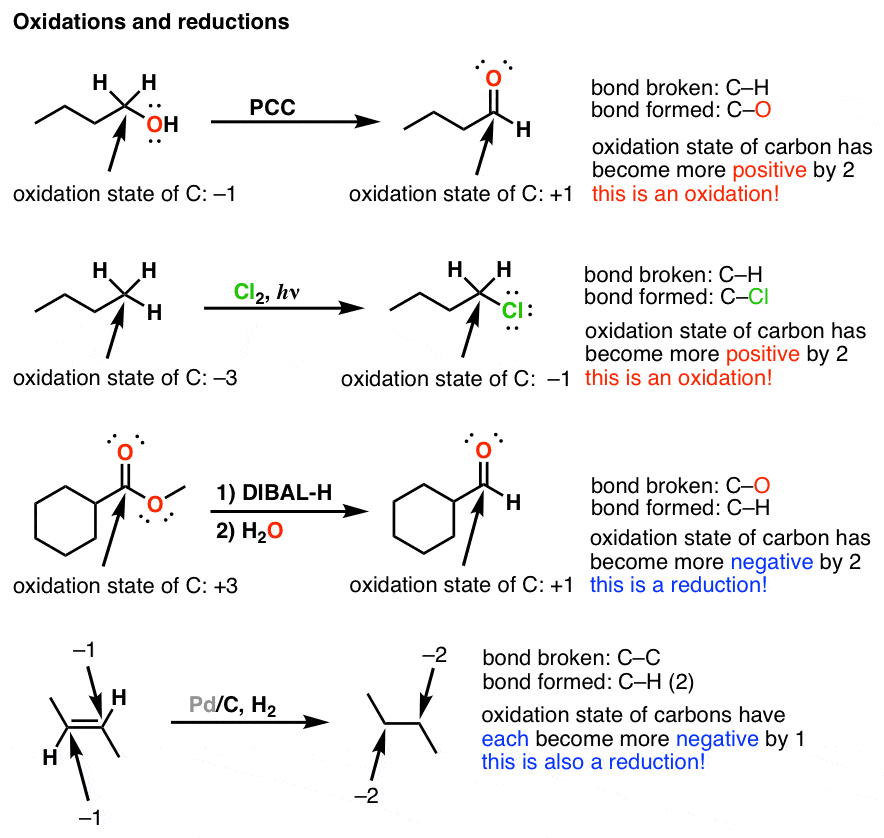
Oxidation And Reduction In Organic Chemistry Master Organic Chemistry
The term oxidation was originally used to describe reactions in which an element combines with oxygen.

. The iron metal is oxidized to form the iron oxide known as rust. When heated iron reacts with oxygen to. The ions combine to form hydrogen fluoride.
Combustion forms the classic example of redox reactions in real-life. Cu 2 2e Cu. Combustion is an example of a redox reaction that occurs so rapidly that noticeable heat and light are produced.
Oxidation is a chemical reaction that occurs in an atom or compound and results in the loss of one or more electrons. H 2 F 2 2 H 2 F 2 HF. 1 0 Al is oxidized Oxidation number.
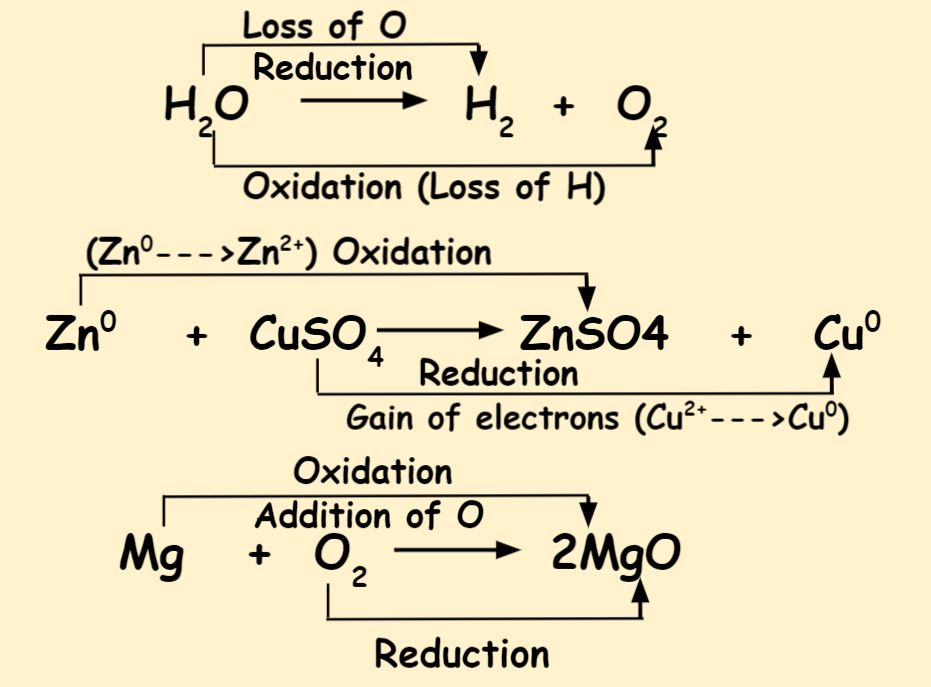
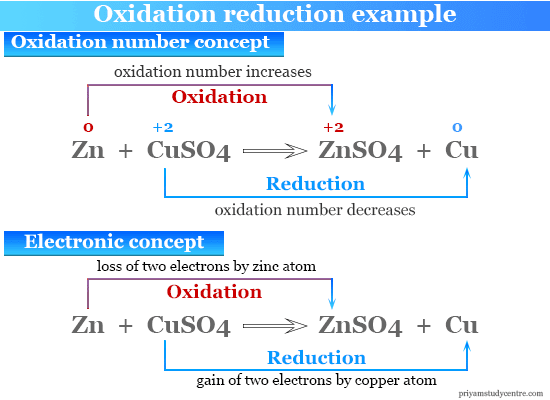
The redox reactions are very common and vital to the basic functions life using in processes such as photosynthesis respiration combustion and corrosion or rusting. Thus copper is displaced from the copper sulfate solution by zinc in a redox reaction. In the examples given above mercury II oxide oxygen and the copper II ion are oxidizing agents and carbon hydrazine.
Electrochemical reactions are great examples of oxidation reactions. More examples include. C O 2 CO 2 oxidation of carbon 2.
2Mg O2 2MgO. Magnesium reacts vigorously with oxygen to produce magnesium oxide. Magnesium oxygen magnesium oxide.
Another example of a redox reaction is the formation of hydrogen fluoride. When a copper wire is placed into a solution that contains silver ions electrons are. The term reduction comes from the Latin stem meaning to lead back Anything that that leads back to.
The tarnishing of silver is just one example of a broad class of oxidation-reduction reactions that fall under the general heading of corrosion. In terms of oxygen transfer. We can break the reaction down to analyze the oxidation and reduction of reactants.
The charge present on any monatomic ion is its oxidation number. In terms of electron transfer. Oxidation reduction reaction can be explained in four different ways.
Metal oxides are bases they react with acids and neutralise them. Addition of electronegative element. Another example is in diamond graphite and buckminsterfullerene carbon have oxidation numbers 0.
Classical Idea of Oxidation and Reduction reactions. For example NitrogenN 2 and hydrogenH 2 in their elemental state will have zero oxidation state. O -2 H 1 C 4.
The above equations are balanced. At first this might look like a simple decomposition reaction because hydrogen peroxide breaks down to produce oxygen and water. 2 H 2 O 2 aq 2 H 2 Ol O 2 g The key to this reaction lies in the oxidation states of oxygen however.
In terms of oxidation number. This reaction is provided below. Corrosion decay and various biological processes are examples of oxidation that occurs so slowly that noticeable heat and light are not produced.
During the process of respiration the carbon-dioxide is reduced whereas the water is oxidised to form oxygen. Fe 2 is oxidized to Fe 3 by hydrogen peroxide when an acid is present. The reactant that brings about the oxidation is called the oxidizing agent and that reagent is itself reduced by the reducing agent.
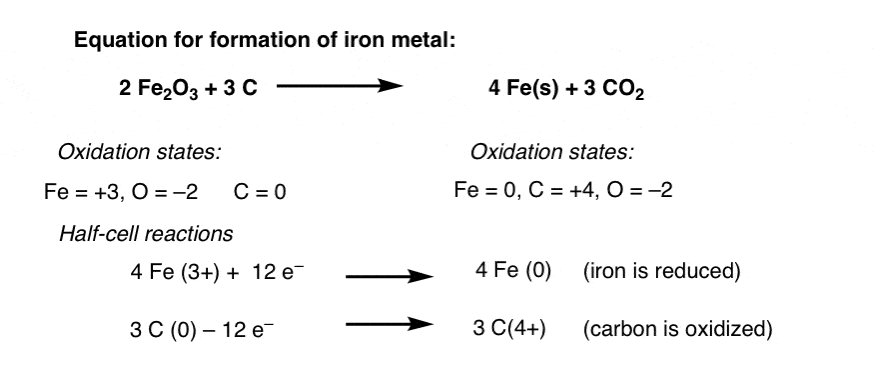
Fe S FeS oxidation of Iron 3. For example the oxidation number of Mg 2 is 2 whereas the oxidation number of Al 3 is 3. Reaction between Iron and Hydrogen Peroxide.
Cu 2 is reduced 2 0 This is not a redox reaction because each element has the same oxidation number in both reactants and products. One real-life example of such a process is the reaction of hydrogen peroxide H 2 O 2 when it is poured over a wound. 2 Fe O 2 Fe 2 O 3.
The oxidation half-reaction can be written as. In terms of hydrogen transfer. Zn Zn 2 2e The reduction half-reaction can be written as.
I hope you find this useful. CH_4 2O_2 - CO_2 2H_2O C_2H_5OH 3O_2 - 2CO_2 3H_2O These and more examples can be found here. The reaction between magnesium metal and oxygen to form magnesium oxide involves the oxidation of magnesium.
Im not a teacher so if there is anything I have missed someone else can help us both out. Another example is the series of reactions that occur when iron or steel rusts. F 2 2 e 2 F the reduction reaction There is no net change in charge in a redox reaction so the excess electrons in the oxidation reaction must equal the number of electrons consumed by the reduction reaction.
Zn is oxidized Oxidation number.
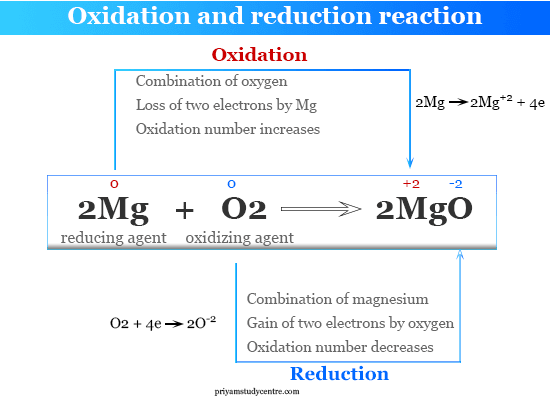
Oxidation Reduction Reaction Definition Concept Examples
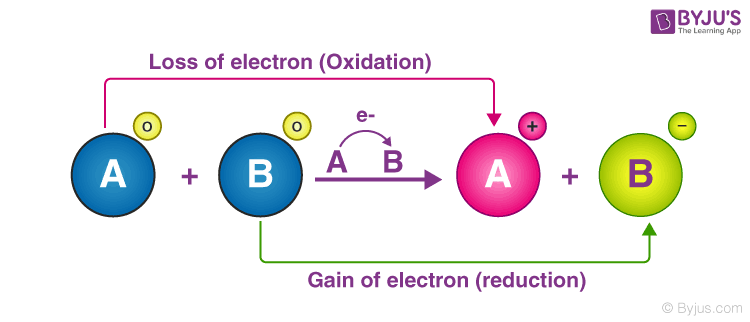
What Is A Redox Reaction Explain With An Example
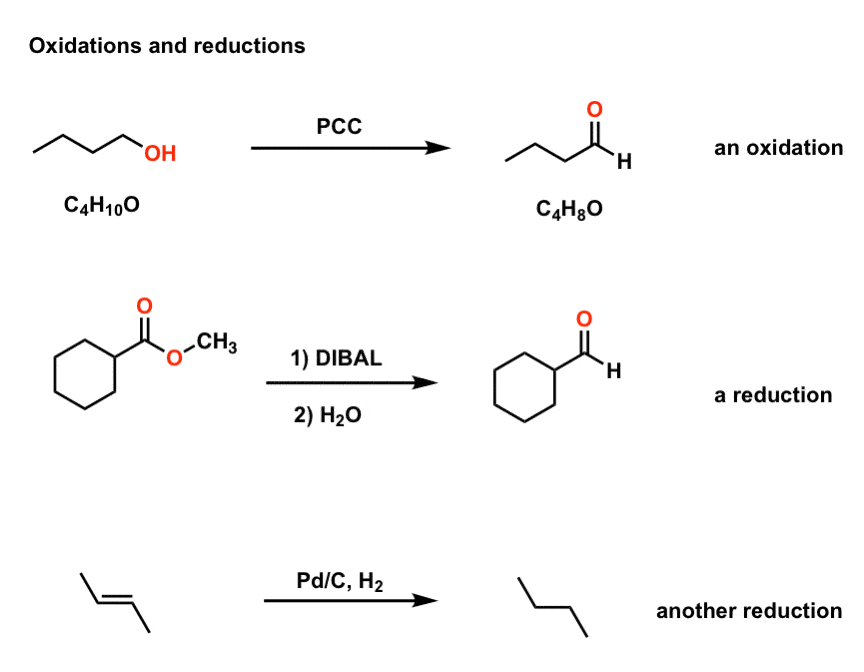
Oxidation And Reduction In Organic Chemistry Master Organic Chemistry

9 Examples Of Redox Reactions In Everyday Life Studiousguy
10 Differences Between Oxidation And Reduction Reaction Dewwool
Oxidation And Reduction In Organic Chemistry Master Organic Chemistry
Oxidation Reduction Reaction Definition Concept Examples

Oxidation Reaction An Overview Sciencedirect Topics

10 Differences Between Oxidation And Reduction Reaction Dewwool
No comments for "List and Describe Three Examples of Useful Oxidation Reactions"
Post a Comment